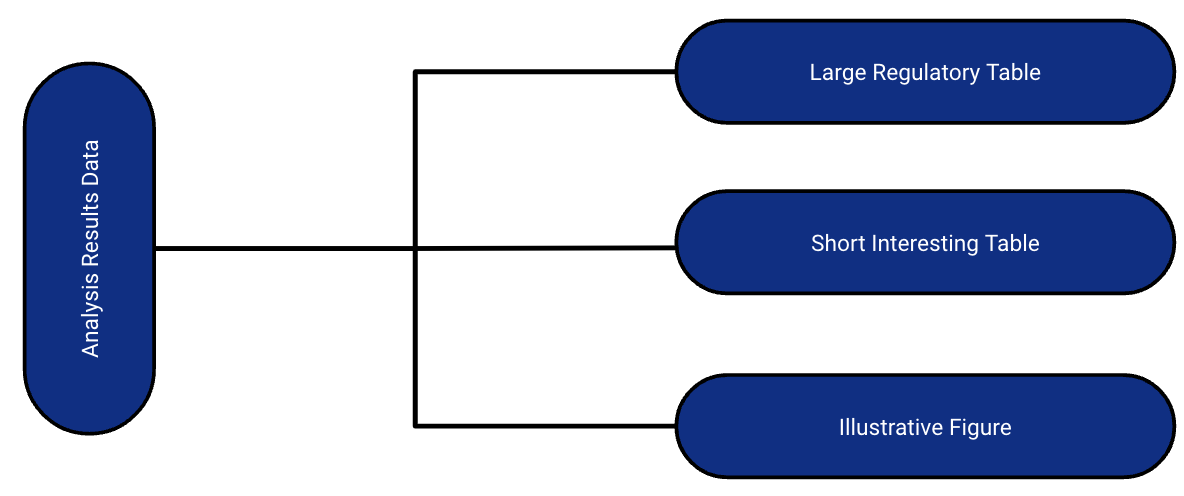
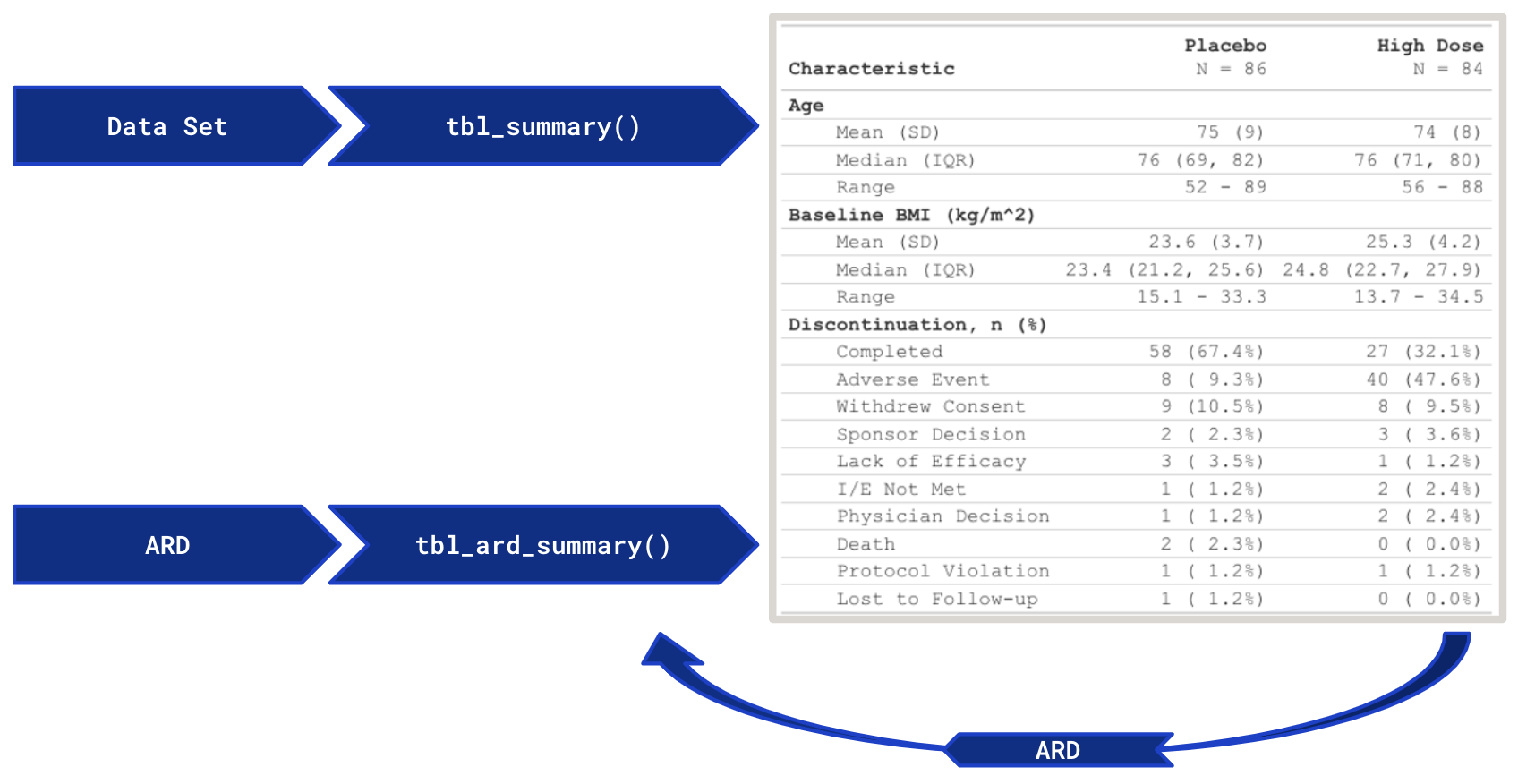
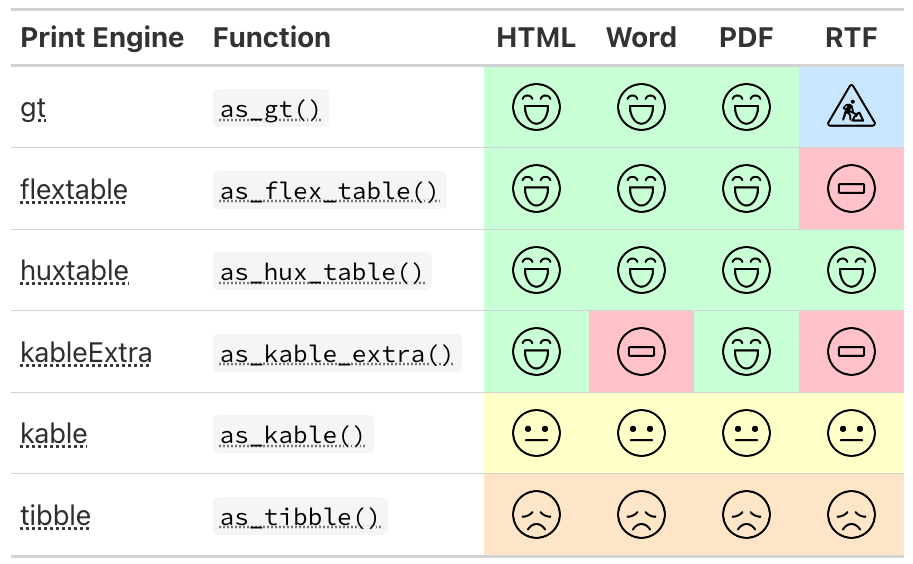
betareg::betareg(), biglm::bigglm(), brms::brm(), cmprsk::crr(), fixest::feglm(), fixest::femlm(), fixest::feNmlm(), fixest::feols(), gam::gam(), geepack::geeglm(), glmmTMB::glmmTMB(), lavaan::lavaan(), lfe::felm(), lme4::glmer.nb(), lme4::glmer(), lme4::lmer(), logitr::logitr(), MASS::glm.nb(), MASS::polr(), mgcv::gam(), mice::mira, mmrm::mmrm(), multgee::nomLORgee(), multgee::ordLORgee(), nnet::multinom(), ordinal::clm(), ordinal::clmm(), parsnip::model_fit, plm::plm(), pscl::hurdle(), pscl::zeroinfl(), rstanarm::stan_glm(), stats::aov(), stats::glm(), stats::lm(), stats::nls(), survey::svycoxph(), survey::svyglm(), survey::svyolr(), survival::cch(), survival::clogit(), survival::coxph(), survival::survreg(), tidycmprsk::crr(), VGAM::vglm() (and more)